38 labeling chemical equations
How to Balance Chemical Equations: 3 Simple Steps The first step in balancing a chemical equation is to identify your reactants and your products. Remember, your reactants are on the left side of your equation. The products are on the right side. For this equation, our reactants are Fe and O2. Our products are Fe2 and O 3. Continuity equation - Wikipedia Continuity equations are a ... species which can be created or destroyed by chemical reactions. ... 4-gradient and μ is an index labeling the spacetime ...
What Are Chemical Equations? - ThoughtCo It's common to indicate the state of matter in a chemical equation by including parentheses and an abbreviation right after a chemical formula. This can be seen in the following equation: 2 H 2 (g) + O 2 (g) → 2 H 2 O (l) Hydrogen and oxygen are indicated by (g), which means they are gases. Water is marked (l), which means it is a liquid.

Labeling chemical equations
Balancing Chemical Equations Flashcards | Quizlet Start studying Balancing Chemical Equations. Learn vocabulary, terms, and more with flashcards, games, and other study tools. How do you Write a Chemical Equation? - A Plus Topper The products of reaction are written in terms of their symbols or molecular formulae on the right-hand side of the equation. A plus (+) sign is added between the formulae of the products. In between the reactants and the products an arrow sign ( ) is inserted to show which way the reaction is occurring. A + B C + D Chemical equation - Wikipedia A chemical equation is balanced by assigning suitable values to the stoichiometric coefficients. Simple equations can be balanced by inspection, that is, by trial and error. Another technique involves solving a system of linear equations . Balanced equations are usually written with smallest whole-number coefficients.
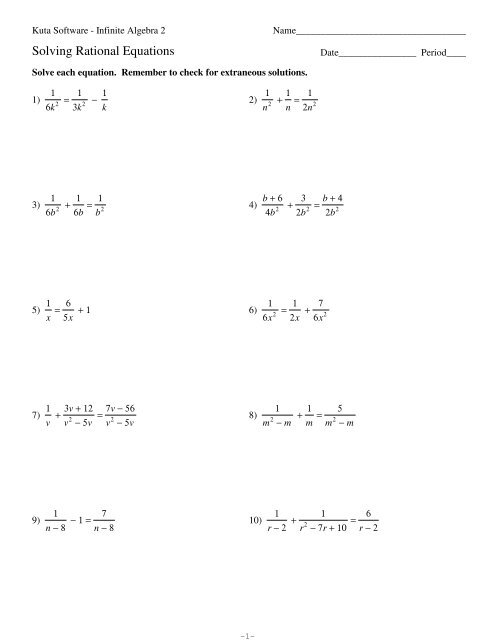
Labeling chemical equations. Balancing chemical equations - How to Balance Chemical Equations Easily ... The unbalanced chemical equation can be written as C3H8 + O2 → CO2 + H2O Step 2 The total number of atoms of each element on the reactant side and the product side must be compared. For this example, the number of atoms on each side can be tabulated as follows. Step 3 How to Number or Label Equations in Microsoft Word Open your document and select your first equation. On the References tab, click "Insert Caption" from the Captions section of the ribbon. In the Caption pop-up window, select "Equation" next to Label. This sets both the word and the number as the caption. Optionally, select a Position for the caption and click "OK" to apply the caption. How to Label Equations in Word: 10 Steps (with Pictures) - wikiHow 1. Open Microsoft Word. It's in the Windows menu (Windows) or in the Applications folder (macOS). 2. Click the Insert tab. It's at the top of the screen (to the right of the Home tab). 3. Click the arrow next to the "Equation" button. It's near the top-right corner of the screen. Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored.
Writing and Balancing Chemical Equations - Course Hero 4 = 4, yes. O. 2 × 2 = 4. (1 × 2) + (2 × 1) = 4. 4 = 4, yes. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. Elementary Principles of Chemical Process - Academia.edu Academia.edu uses cookies to personalize content, tailor ads and improve the user experience. By using our site, you agree to our collection of information through the use of cookies. Definition of dissolve and the state label (aq) in chemical equations C O X 2 ( a q) would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). B r X 2 ( a q) would imply homogeneous solution of liquid bromine in water. N a C l ( a q): Salt dissolved in water. A colloid is a heterogeneous phase. We can start by labeling our chemical equations.docx - Course Hero View We can start by labeling our chemical equations.docx from BIOLOGY 171 at Dekalb High School. We can start by labeling our chemical equations: Final Equation: W (s) (s) ΔHrxn = ? Equation 1: 2 W
How do you label a chemical equations? - Answers What are chemical equations? chemical equations are a formal algebra that permits the calculation of chemical reactions. What are Chemical Equations? Detailed Explanation, Examples A few examples of chemical equations are listed in bulleted text below. PCl5 + 4H2O → H3PO4 + 5HCl SnO2 + 2H2 → 2H2O + Sn TiCl4 + 2H2O → TiO2 + 4HCl H3PO4 + 3KOH → K3PO4 + 3H2O Na2S + 2AgI → 2NaI + Ag2S How do you write chemical formulas? There's a particular way of writing what's in a molecule called a chemical formula. Labeling A Chemical Equation Part 2 - YouTube The instructions for labeling a chemical equation. Symbols in Chemical Equations - Harper College Symbols in Chemical Equations. Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read used when the reaction can proceed in both directions - this is called an equilibrium arrow and will be used later in the ...
Balancing chemical equations (how to walkthrough) (video) - Khan Academy N2 + H2 -> NH3. On the left there is 2 N and 2 H. On the right there is 1 N and 3 H. If we tried to balance starting with H you'd need to use a fraction or decimal and would get messy, so let's start with N. There's 2 on the left and 1 on the right, so we need to change the coefficient of NH3 to 2.
Chemical Equations - Let's Talk Science Shown is a chemical equation for the breakdown of sulphuric acid in the presence of heat. On the left side is the equation for the chemical formula for sulphuric acid, H 2 SO 4. On the right side of the equation are the chemical formulas for sulphur trioxide, SO 3 and water, H 2 O. Between the two sides is an arrow pointing from left to right.
Balancing redox equations - Practice exercises There are two common techniques for balancing redox equations: oxidation number change method ion-electron method (also called the half-reaction method). Periodic table of the elements Chemistry calculators
Symbols used in Chemical Equations Flashcards | Quizlet used for reversible reactions. +. used to separate two reactants or two products. ∆ (over the reaction arrow), indicates the application of heat to the reactants. ↓. indicates a precipitate formed by a reaction. (aq) designates an aqueous solution; the substance is dissolved H₂O; placed after the chemical formula.
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Start by writing the chemical equation in terms of the substances involved: C 2 H 6 + O 2 → CO 2 + H 2 O We have two carbon atoms on the left, so we need two carbon dioxide molecules on the product side, so that each side has two carbon atoms; that element is balanced.
How to Write a Chemical Equation (with Pictures) - wikiHow In a basic double replacement equation you will have 2 cations and 2 anions. The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. [11] For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2
Labeling of chemical containers - periodni.com Fine-adjust the print position: Shift from left: mm. Shift from top: mm. How to print labels? In your browser click the Page Setup in the File menu (hit ALT-F if menu isn't visible). Set the Orientation to portrait and the Scale to 100%. Un-check the "Shrink to fit page width" box and check the "Print background (colors and images)".
How to Write Chemical Equations - YouTube Mr. Causey shows you how to WRITE chemical equations. Mr. Causey discusses the parts of a chemical equation, the symbols involved and the steps required to w...
Examples of Balanced Chemical Equations - ThoughtCo To write a balanced equation, the reactants go on the left side of the arrow, while the products go on the right side of the arrow. Coefficients (number in front of a chemical formula) indicate moles of a compound. Subscripts (numbers below an atom) indicate the number of atoms in a single molecule.


Post a Comment for "38 labeling chemical equations"