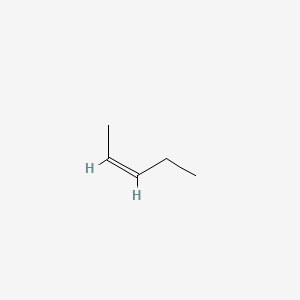
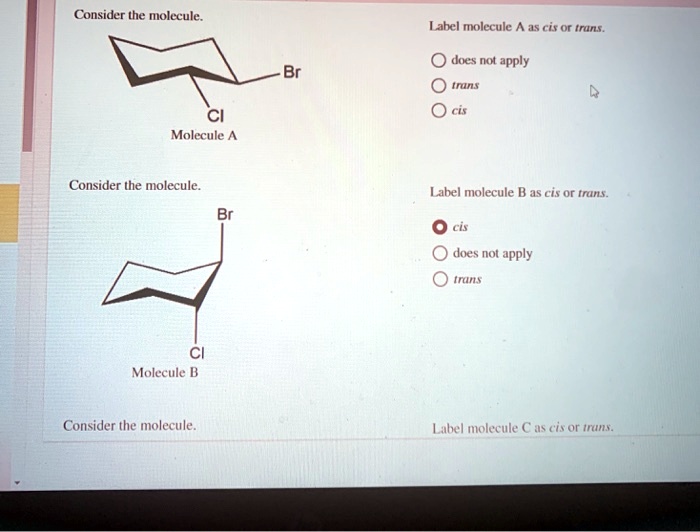
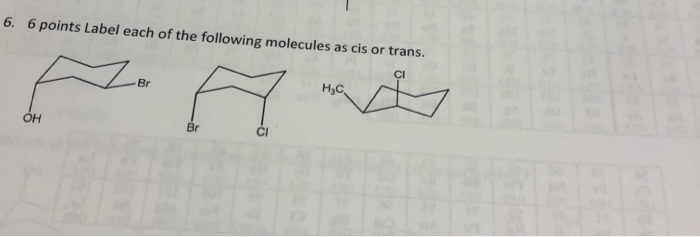
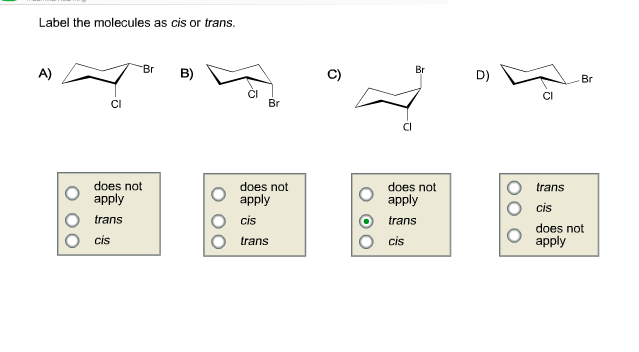
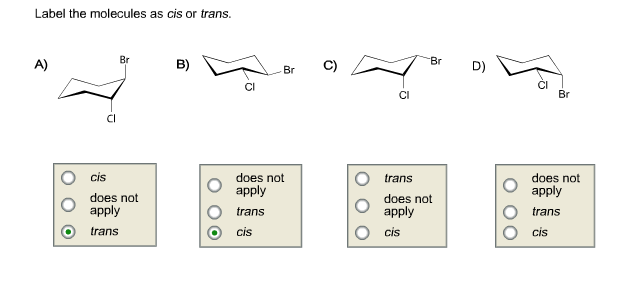
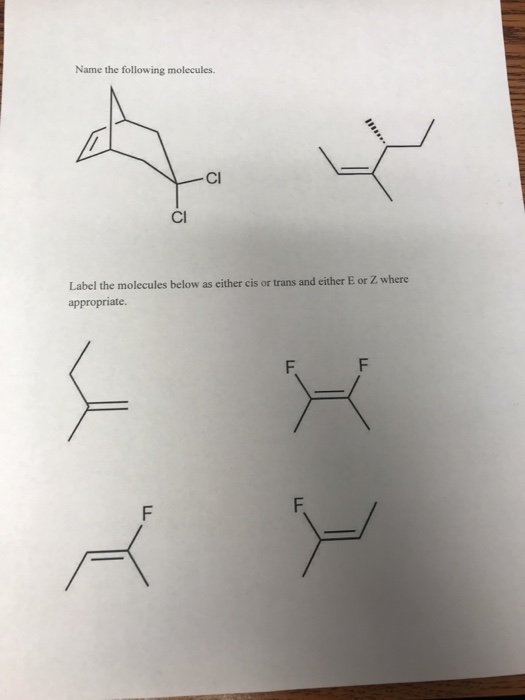
39 label the molecules as cis or trans.
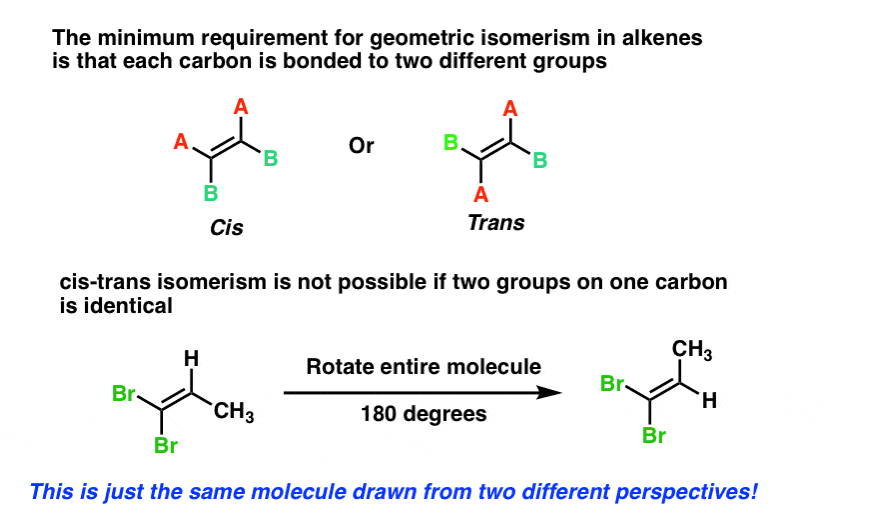
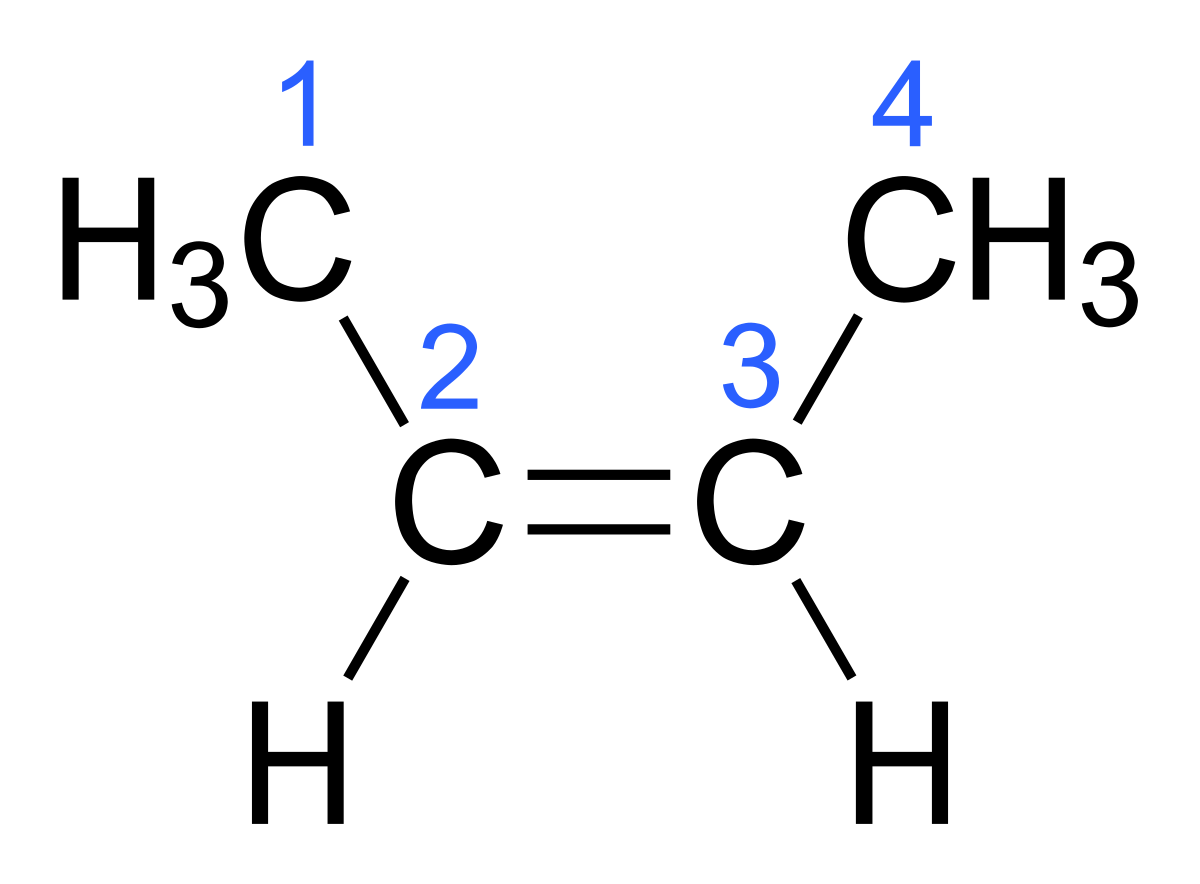
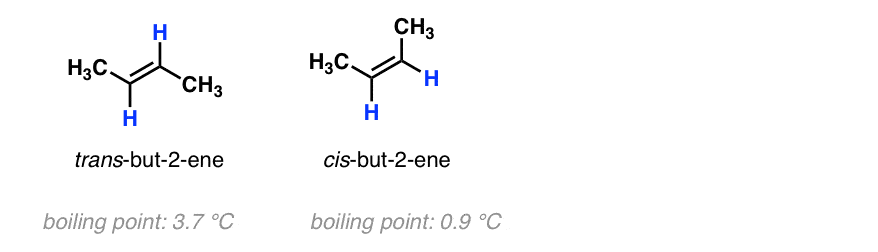
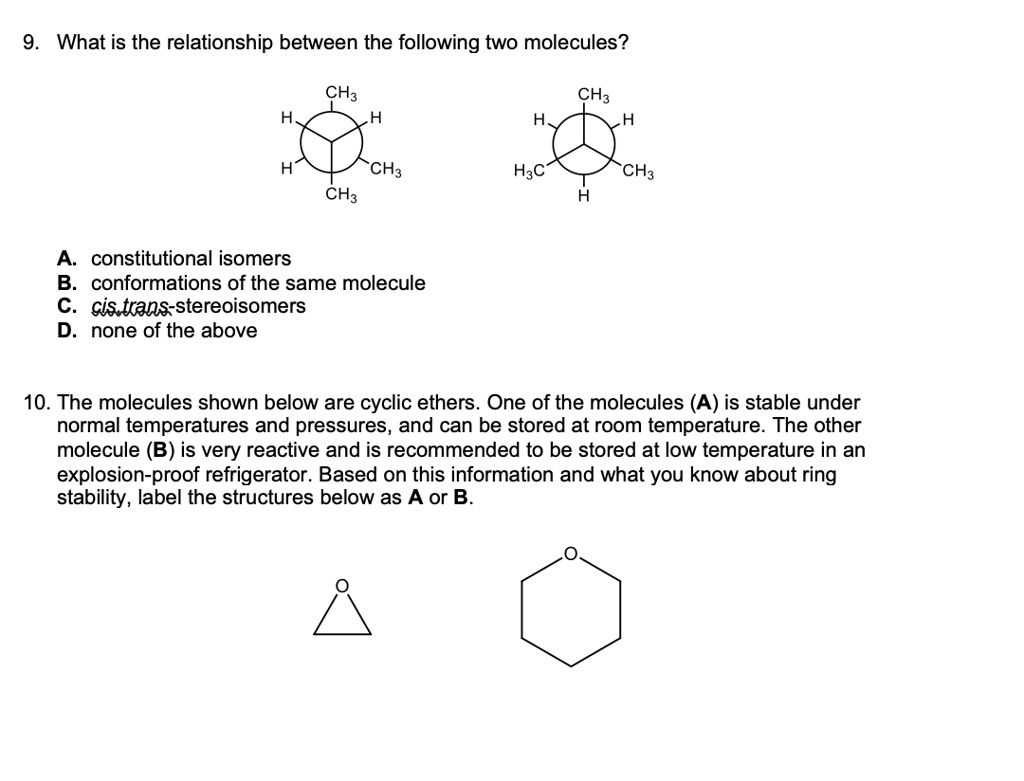
Chapter 4 Flashcards | Quizlet trans-1-tert-butyl-4-ethylcyclohexane or trans-1- (1,1-dimethylethyl)-4- ethylcyclohexane Provide IUPAC names for the structure. cis-1-sec-butyl-2-ethylcyclopentane Name this structure. 3-cyclobutylpentane Name this structure. 1-chloro-2-isopropylcyclopentane Name this structure. 3,5-dicyclohexylnonane Name this structure. a and d Cis and Trans - Organic Chemistry | Socratic The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon, as in but-2-ene. Then the two identical methyl groups are either cis or trans to each other, and the two identical hydrogen atoms are either cis or trans to each other.
Geometric Isomerism Cis- and Trans- Mean in Chemistry - ThoughtCo When two substituent atoms or groups bend in the same direction, the molecule is prefixed by cis-. This molecule is cis-1,2-dichlorocyclohexane. Trans-Alicyclic Compounds Todd Helmenstine This molecule has the substituent chlorine atoms bending in opposite directions or across the plane of the carbon-carbon bond.

Label the molecules as cis or trans.
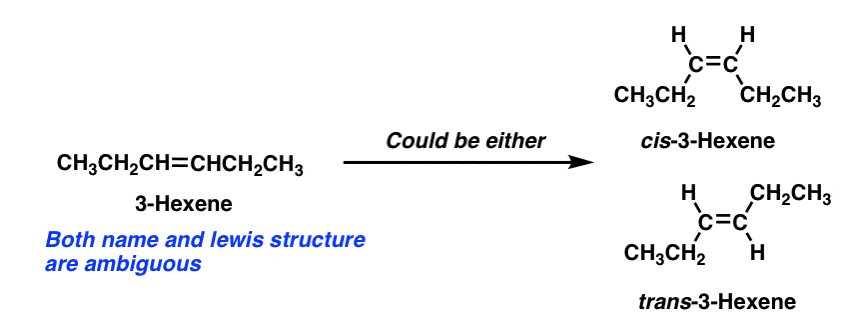
What are Cis and Trans Double Bonds? That's Simple! - Quirky Science If a molecule has more than one double bond, the molecule can have both cis and trans bonds. For example, 3,4-dimethyl-hexa-2,4-diene, with two, has one cis-double bond and one trans-double bond (see topmost image). The difference between cis and trans is not merely of intellectual value. Life chemistry requires very specific chemical structures. Chapter 4 Biol 189 Homework Flashcards | Quizlet first, third and fourth. Since carbon atoms are tetravalent (able to form four bonds), atoms may branch off a carbon atom in as many as four places. The ability of a carbon atom to form four different bonds allows carbon to form many different sizes and types of molecules. Carbon atoms may form chains, rings, or combinations of chains and rings. Cis-Trans Isomers - Definition, Detailed Explanation with Examples - BYJUS The cis and trans isomers of butenedioic acid display very different reactivities, which can be attributed to the difference in their properties. Maleic acid is the cis isomer and fumaric acid is the trans isomer. Elaidic acid and oleic acid are cis-trans isomers.
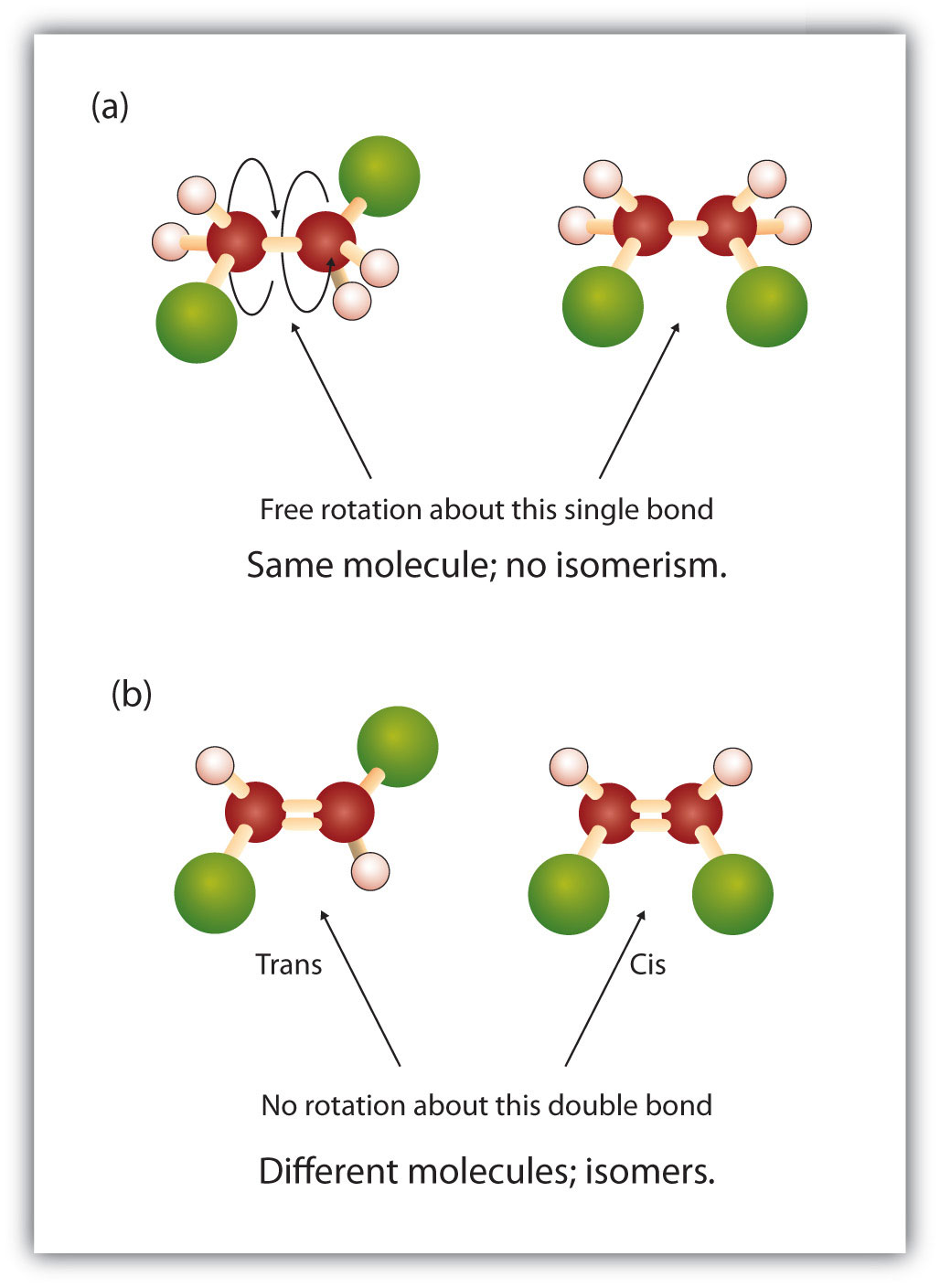
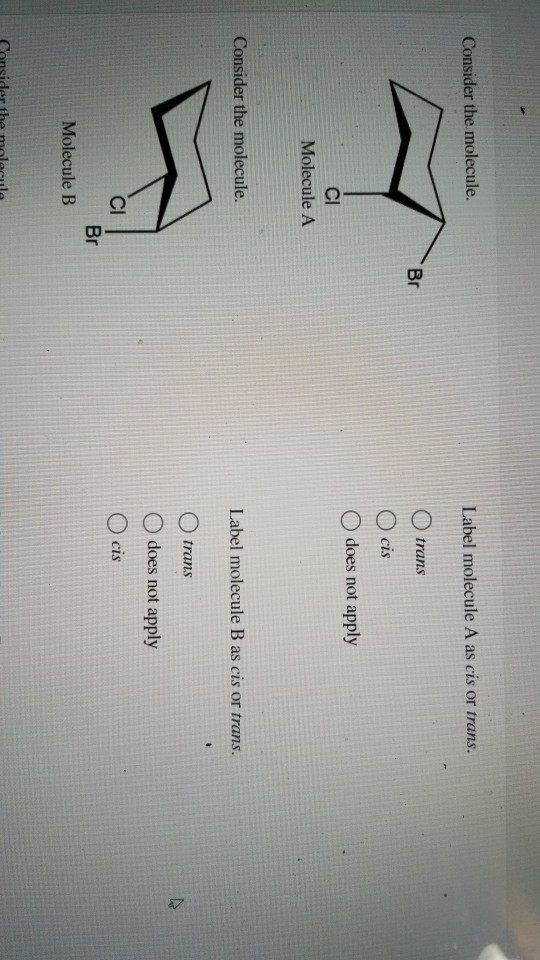
Label the molecules as cis or trans.. Difference Between Cis and Trans Isomers - Isomerism - BYJUS Due to loosely packed molecules, cis isomers have relatively lower melting points than trans isomers. Due to tightly packed molecules, the melting points of trans isomers are usually higher than those of cis isomers. The boiling point of cis isomers is high due to the presence of strong forces of attraction between the atoms of the cis isomer. CHEM 2443 Final Flashcards | Quizlet trans Label molecule D as cis or trans. trans For the substituted cyclohexane compound shown, identify the atoms that are cis to the hydroxyl (OHOH) substituent. B, D, G, I, K Complete the most stable chair conformation of cis‑1‑bromo‑3‑chlorocyclohexane by filling in the missing atoms. Cis-trans isomerism (video) | Khan Academy Cis and trans molecules do not freely interconvert between each other. However, some interconversion will usually occur due to the dynamic equilibrium of a reaction. After a trans bond is formed the reverse reaction may occur (remaking the reactant) and then the reactant could undergo the reaction again but this time forming the cis bond. 1 comment Cis-Trans Isomerism in Alkenes | MCC Organic Chemistry - Lumen Learning It is particularly important that you make molecular models of some simple alkenes to gain insight into the geometry of these compounds. Geometric isomerism (also known as cis-trans isomerism or E-Z isomerism) is a form of stereoisomerism. Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms ...
Cis-Trans Isomers - Definition, Detailed Explanation with Examples - BYJUS The cis and trans isomers of butenedioic acid display very different reactivities, which can be attributed to the difference in their properties. Maleic acid is the cis isomer and fumaric acid is the trans isomer. Elaidic acid and oleic acid are cis-trans isomers. Chapter 4 Biol 189 Homework Flashcards | Quizlet first, third and fourth. Since carbon atoms are tetravalent (able to form four bonds), atoms may branch off a carbon atom in as many as four places. The ability of a carbon atom to form four different bonds allows carbon to form many different sizes and types of molecules. Carbon atoms may form chains, rings, or combinations of chains and rings. What are Cis and Trans Double Bonds? That's Simple! - Quirky Science If a molecule has more than one double bond, the molecule can have both cis and trans bonds. For example, 3,4-dimethyl-hexa-2,4-diene, with two, has one cis-double bond and one trans-double bond (see topmost image). The difference between cis and trans is not merely of intellectual value. Life chemistry requires very specific chemical structures.















Post a Comment for "39 label the molecules as cis or trans."